The emergency use of Corona vaccine in the country has cleared its way. On Friday, the Expert Committee of the Central Government recommended approving the emergency use of Covishield, the vaccine of Oxford-AstraZeneca. According to the information, the drug controller can get final approval on Saturday. 5 crore doses of Covishield are ready.

They will be airlifted to regional centers across the country in a day or two. The central government has completed the preparations to start a nationwide vaccination campaign from 6 or 7 January. The states have handed over the lists of the one crore health workers who will get the vaccine first.
Covishield is being built by the Serum Institute of India (SII), Pune. It was applied for permission to use in India on 6 December. Earlier in the UK, this vaccine from Oxford-AstraZeneca has been approved.
This is how the vaccine will reach us
1 crore health workers will get vaccinated by February, 2 crore front line workers by March
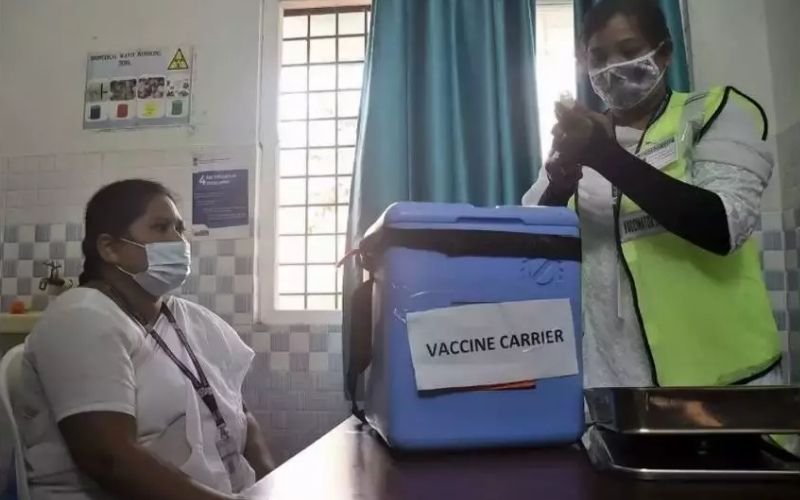
Currently the vaccine is in Himachal
First, the government will buy the vaccine from the Serum Institute. 5 crore vaccine doses have been approved by Himachal’s Central Drugs Laboratory. Serum will also be responsible for transporting them to the regional centers from there. After that they will reach the state headquarters. States will then start vaccination according to their strategy.
List of 1 crore people ready
First of all, one crore health workers will get vaccinated. All states have sent their lists to the Center. They will be vaccinated by February. After that, frontline workers like police, municipal employees will get vaccinated. Their number is close to 20 million. The center has also sought their lists. They must have been vaccinated by March.
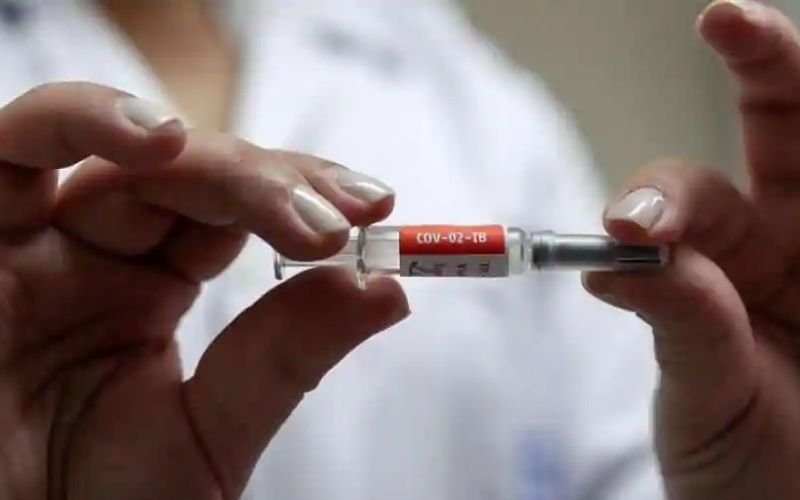
Covishield effective up to 70%
In clinical trials, Covishield was found to be up to 90% effective. However, trials in humans resulted in different results in Britain and India. Now the central government believes that it is effective up to 70% i.e. it will provide protection from corona to 70 people out of 100.

E-Win Watch on Vaccine Movement
The complete information about which batch vaccine dose has reached the cold chain will be updated on e-Win (Electronic Vaccine Intelligence Network). With this, complete monitoring will be done.

There are 6 different vaccines in the country, all will be in the market by August
- Coveshield: 10 million doses this month
Approval is on the way. 5 crore doses are ready. By this month 10 crore doses will be made. This will be the first vaccine to be introduced in the country. - Covaxin: clearance possible in February
Bharat Biotech is formed by the National Institute of Virology (NIV) and the Indian Institute of Medical Research (ICMR). Third phase trials are underway in 25 cities. No volunteers have shown any side effects so far. - Sputnik-V: Approval possible by March
Russian vaccine. Dr. Reddy’s Laboratories and RDIF are conducting Phase-1/2 trials in India. 700 rupees Per dose will be received. - Jayakov-D: Will come after March
Zydus Cadila. Phase-2/3 trials are running simultaneously. The price is not fixed. May come anytime after March. - Biological E: possible by July
It is formed by American company Dynavax Technology and Baylor College in Houston. Phase-2 trials have started in India. This vaccine will be available by July. - HGCO-19: possible in August
Geneva Pharma and HDT Biotech of Pune. Trials on humans have not started yet. It may hit the market in August.

Apart from these, Pfizer’s vaccine has been approved for emergency use in the UK. Applied for approval in India. However, this was not discussed in the expert committee meeting on Friday.
Indigenous vaccine awaiting approval: Bharat Biotech’s vaccine claim of cocaine was also discussed by the expert committee. The committee asked the company to register faster and more volunteers for the trial.
Also, read our Blog on “COVID19 Army ready for Corona’s vaccination campaign” Click here.


